Overview
Understanding DTRA: Priorities, Initiatives, and Involvement

DTRA's Mission & Vision
DTRA's vision is to make research participation accessible for all.
DTRA’s mission is to accelerate adoption of innovative research methods, including decentralized elements, by collaborating with a community of global stakeholders.

DTRA's Priorities & Initiatives:
Why They Matter
Decentralized trials can benefit patients, researchers, and the development of new treatments through improved patient experience, more efficient research processes, and potential cost savings. DTRA's priorities and initiatives focus on advancing decentralized trials and research, which are crucial for enhancing patient access and experience, while reducing barriers to participation in clinical studies. Our goal is to promote decentralized trials by establishing clear goals and recommendations, such as defining common nomenclature, promoting best practices, and facilitating education and information sharing. Through these efforts, we seek to improve decentralized trials and support implementation and adoption, ultimately leading to more inclusive, accessible, and patient-centered clinical studies.

What Are the
Priorities and Initiatives?
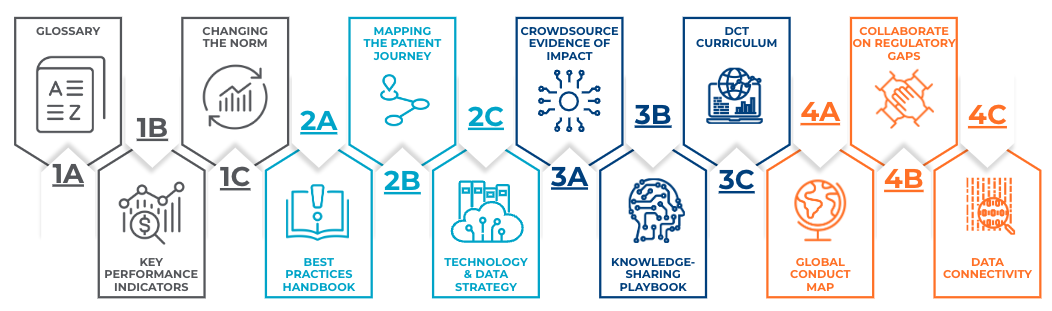
The founding members of DTRA established four key priorities to guide DTRA's efforts toward realizing its collective mission of facilitating the adoption of patient-centric, decentralized clinical trials and research. These priorities focus on developing practical solutions that can drive meaningful change across the field.
Within the four Priorities, 12 Initiatives were established to dive deeper and create a roadmap to develop strategic solutions through collaboration.

Who is Involved?
DTRA has over 100 organizational members and many more individual members from varying industries. These include life sciences and research companies, research institutions and sites, technology and service providers, patient advocacy groups, regulatory agencies, and many more.
DTRA is closely partnered with other key cross-industry groups driving change, including CTTI, Transcelerate, IMI, ACRP, SCRS, ACRO and ATA.
Together we aim to support the adoption of new methods that will help modernize how patients interact with clinical trials in the future.
Explore the partner organizations that collaborate to drive innovation and progress in the life sciences and clinical trials ecosystem.
How Can You
Get Involved?
- Get informed about DCTs and best practices
- Become part of the discussion. Attend DTRA events, webinars and conference sessions where DCTs are discuussed. Add your voice to the collaboration!
- Join DTRA members speaking at conferences, attend the DTRA TGIF-DCT Clubhouse, or listen to our podcast
- Join DTRA to contribute to making research accessible to everyone and shaping the future of clinical research while enhancing the patient experience