1572 Co Lab
Rare Disease Patient Journey - Scenario for PI Oversight

Scenarios for PI Oversight and Delegation of Trial Related Activities
DTRA chartered cross-functional teams to create processes and tools to support the adoption of DCT trial design and execution. This example will show how these methods could be used in an Rare Disease Clinical Trial using decentralized methods.
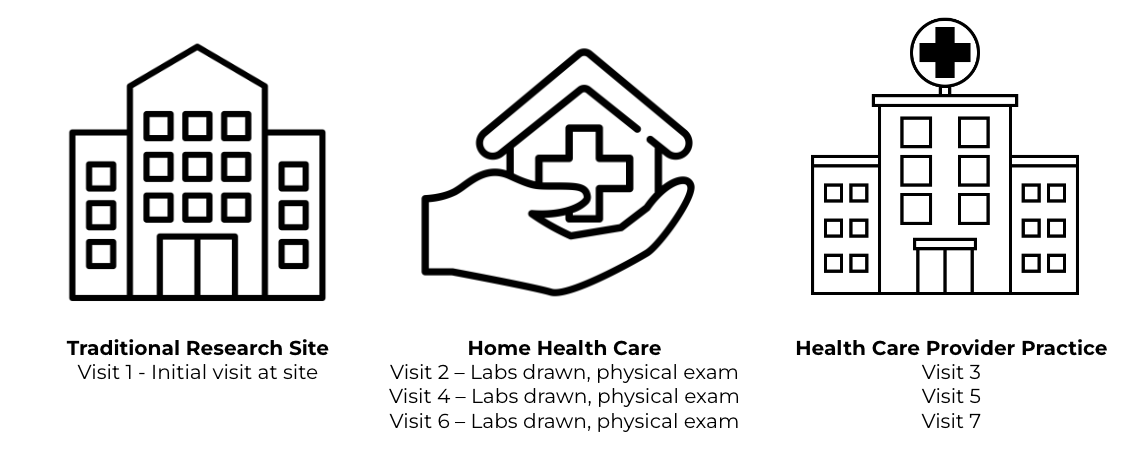


Rare Disease Patient Journey Map

Rare Disease Sample Protocol Information

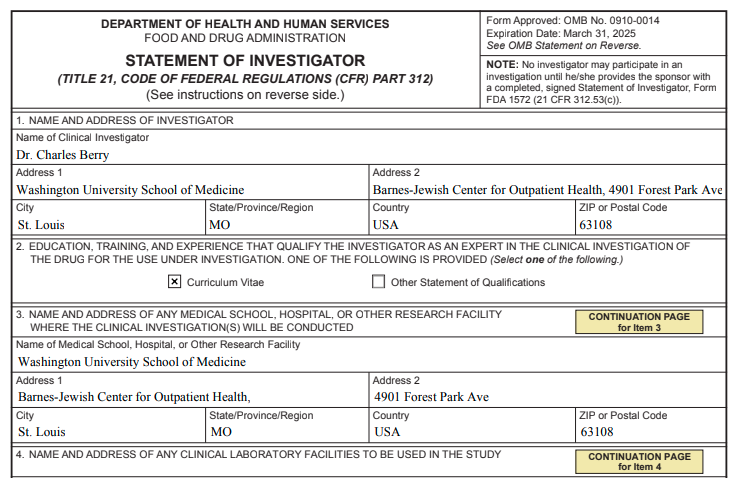

Completed 1572 Form and Additional Page for Rare Disease Site per Briefing Document

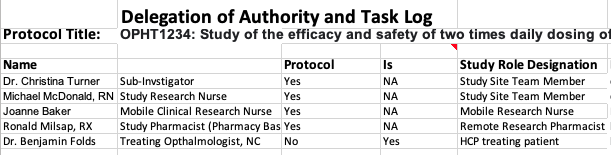
Combined Delegation of Authority and Task Log for Rare Disease Trial Site per Briefing Document