Roles & Responsibilities CoLab
Site Adoption

Roles & Responsibilities
Key Questions & Decisions Instructions
Decision elements to determine appropriate documentation of delegated trial-related activities
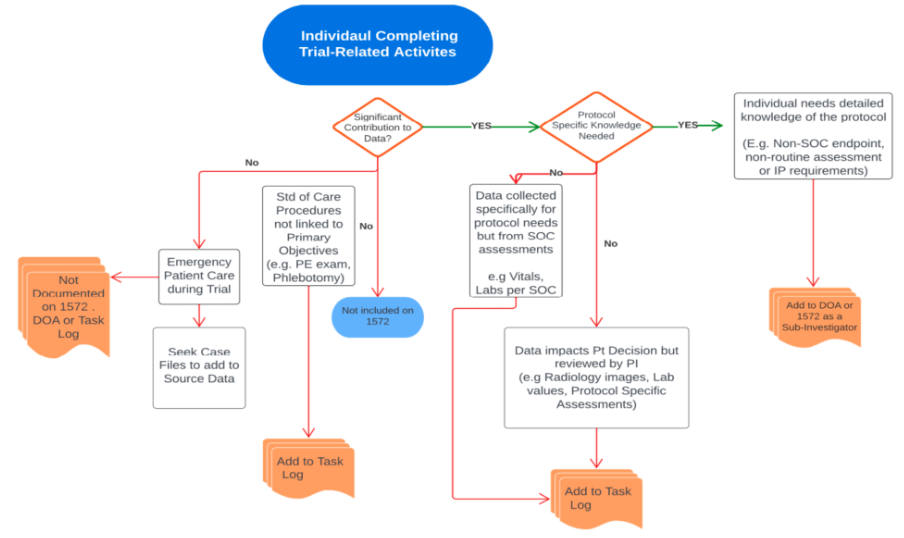

A Guide for Stakeholders with Timing
Instructions
1. These tools were created with the intention to increase clarity and coordination across roles performed by different organizations and individuals using DCT elements in trials.
2. Sponsors: Use these tools while writing protocols including DCT elements, when identifying sites, and during study start up / conduct.
Suggested Sponsor Users: Clinical Operations Leaders, Medical Team, Site Engagement / Feasibility Team, Digital Health Team
Research Site Staff: Use these tools when considering participation in as a research site and as part of trial start up and conduct.
Suggested Site Users: Clinical Research Coordinator, Site Management / Leadership, Resource and Budget /Contract Managers
Technology and service providers: Use these tools when planning to support a specific clinical trial, and when defining what is needed to support any clinical trial.
Suggested Service Provider Users: Implementation and Delivery Leaders, Technical Support Team, Training Team
3. Start at the Platform Card - Answer these questions first, alone or in collaboration between sites, CROs, Sponsors and Service providers.
Check the Box for any of the DCT elements that apply in your specific clinical trial to be directed to the relevant cards.
4. Use the questions as an approach to set clear mutual expectations about who is doing what, what is being used, and how the DCT element impacts the study conduct and data flow.
Assumptions
1. These tools are used in alignment to meet ICH, GCP requirements and local regulatory guidance recommendations including GDPR.
e.g. Conducting Clinical Trials With Decentralized Elements (Sept 2024)
2. Any DCT technology solutions meet technical requirements for use in clinical trial conduct (CFR 11, GDPR, etc). and have been qualified for use by Sponsor
3. All DCT elements will be conducted within state laws
4. The PI will be informed when any of the DCT elements are used as part of the site's study conduct and or oversight
5. These tools were designed to for use when DCT elements are included in the protocol design, rather than when the protocol adapts to use them following study start
Recommendations
1. As a research site, consider using these tools to better define what is needed to use DCT elements efficiently at your site.
We suggest you use these questions as the basis of a readiness assessment for each DCT element by reviewing the questions to be answered and the capabilities required. This may also help clarify to sponsors what would be needed to adopt the DCT element successfully.
2. Use the associated excel spreadsheet to align your specific study operational plan (DCT Elements, Vendor plan) and team model to a clarify roles and responsibilities in a specific study.
